carboxylate ir stretch
Free carboxylate stretching modes J Phys Chem A. This region corresponds to the stretching modes of the carboxylate group and is often.

Infrared Spectrum Of Ethanoic Acid Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Ethanoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes
The weight of the IR band area using the COO antisymmetric stretch vibration at 1580 cm 1.
. Modeling the IR spectra of aqueous metal carboxylate. Gas-phase spectra of a series of benzoate anions have been recorded and compared to condensed-phase spectra revealing the profound influence of th. Spectra were recorded in regions below 800 cm 1 8001500 cm 1 the fingerprint region the region between 2800 and 3000 cm 1 C-H stretch region and finally the region between 3000 and 3600 cm 1 O-H stretch region by method as mentioned by Belton et al.
To divide our two regions we draw a line around 3000 here. We report the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions. We have tested this principle with ab initio modeling for aqueous metal carboxylate complexes and.
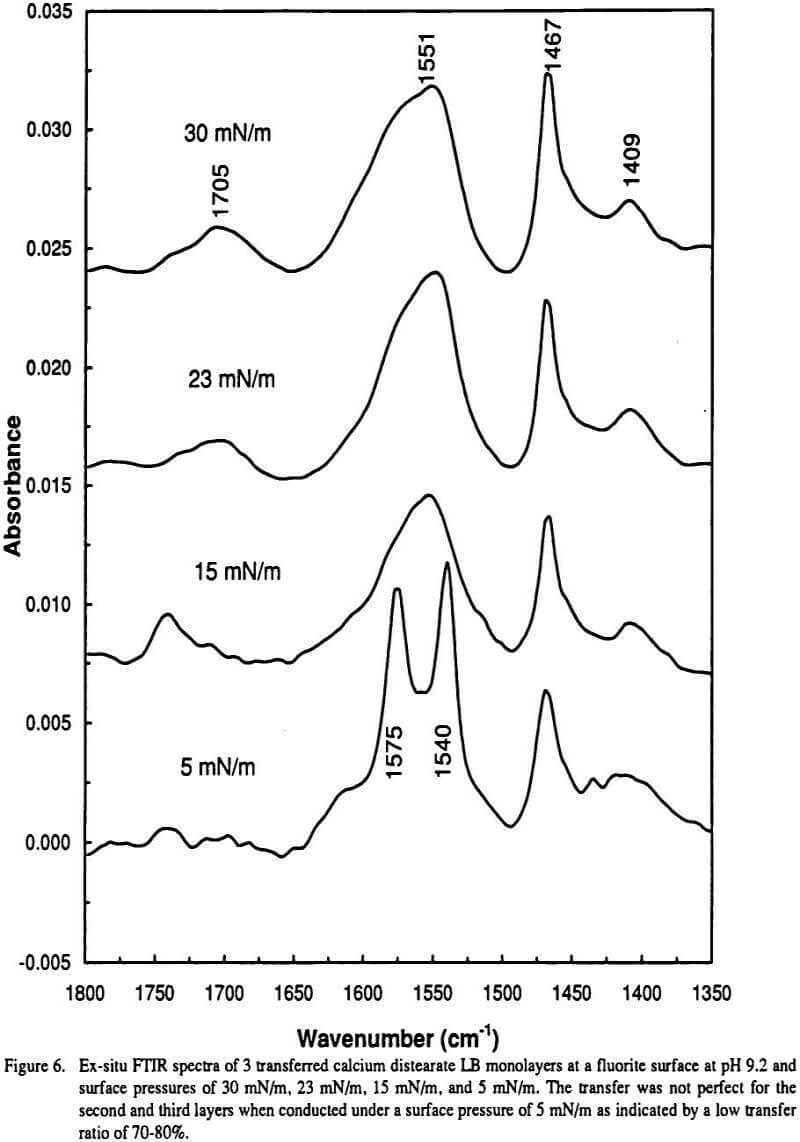
Deconvolution of the IR carboxylate stretch frequency region 1525-1675 cm-1 in the three cytochromes yield different vocoa distributions. Carboxylate groups associated with calcium ions in three-dimensional seven- or eight-fold coordination which may contain carboxylate groups coordinated with calcium ions in different modes ie unidentate and bidentate modes are responsible for the IR doublet at 1540 and 1575 cm 1. The asymmetric and symmetric stretching.
Abstract A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is monodentate or bidentate. So we know this is our carbon-hydrogen in here. A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is monodentate or bidentate.
In fact different types of attachments like ionic and coordination bonds monodentate bidentate bridging and bidentate chelating can be interpreted by observing antisymmetric stretching of. The stretching vibration of the carboxylate is known to have a wide variation in position due to hydrogen bonding and therefore the assignment of the ν COO modes had to be made carefully. Download scientific diagram FT-IR spectra.
Lets draw a line around 1500. A linear standard curve using anhydrous sodium salicylate has been obtained from the plot of carboxylate COO concentration vs. Weatherability of pastel and dark colored rigid PVC compound can be achieved by substituting the more light.
We have tested this principle with ab initio modeling for aqueous metal carboxylate complexes and have shown that it does indeed hold. The infrared spectra of six aqueous carboxylate anions have been calculated at the m05-2xcc-pvtz level of theory with the smd solvent model and validated against experimental data from the literature over the region of 1700 cm -1 to 1250 cm -1. This was especially true for the compounds II III V and VI containing pap mpcm pcb nia ligands in addition to the carboxylate.
So lets look at an IR spectrum just a generic IR spectrum for an acid and hydride. Carboxylate stretches in phenothiazine hydrochlorides and phenothiazine ILs. ProIbu CProIbu PrmIbu and.
In the case of the bonito cytochrome c carboxylates two vocoa populations are clearly distinguishable in the deconvoluted spectra--which is not the case for the more complex vocoa deconvolutions of the other two. Infrared IR spectroscopy is a useful tool for determining the speciation of metal loaded organic phases. This assignment is in agreement with the observed IR doublet for bulk.
The carbonyl stretching bands were the most distinctive IR features to distinguish the carboxylic acid group from the conjugate carboxylate moiety 41 42. Carboxylate anions exhibit characteristic vibrational spectra in the infrared IR which typically includes an intense peak due to symmetric stretching of the COO functional group as well as a peak due to antisymmetric stretching. In the previous work relationships of the form V-10αAνMβAνD were obtained for the.
Finally lets look at one more example of a symmetric and asymmetric stretch and thats an acid and hydride.
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts

How Range Peak Ir Spectrum For Carboxylic Acid Salts Ex Calcium Lactate

Ir Spectra Of Indole 3 Acetic Acid In Kbr Indole Nh Band At 3389 Cm 1 Download Scientific Diagram

Infrared Spectrum Of Benzoic Acid C7h6o2 C6h5cooh Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Benzoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes
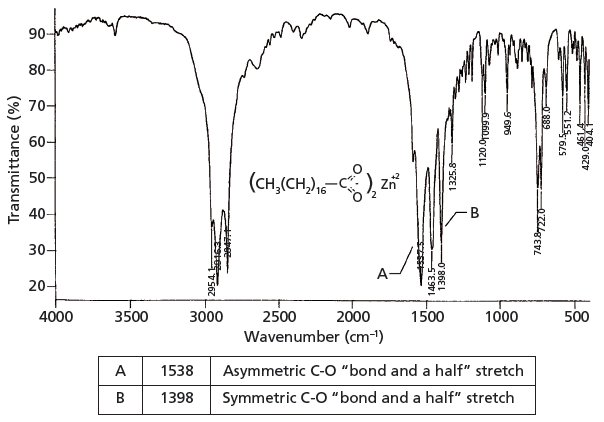
The Carbonyl Group Part V Carboxylates Coming Clean
21 3 Spectroscopy Of Carboxylic Acids Chemistry Libretexts

Ir Infrared Absorption Bands Of Carboxylate

How Range Peak Ir Spectrum For Carboxylic Acid Salts Ex Calcium Lactate

Interpretation Of Carboxylic Acid Ir Spectra Youtube
The C O Bond Part Iii Carboxylic Acids
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts

Lec15 Ir Spectra Of Carboxylic Acids Amides And Esters Youtube
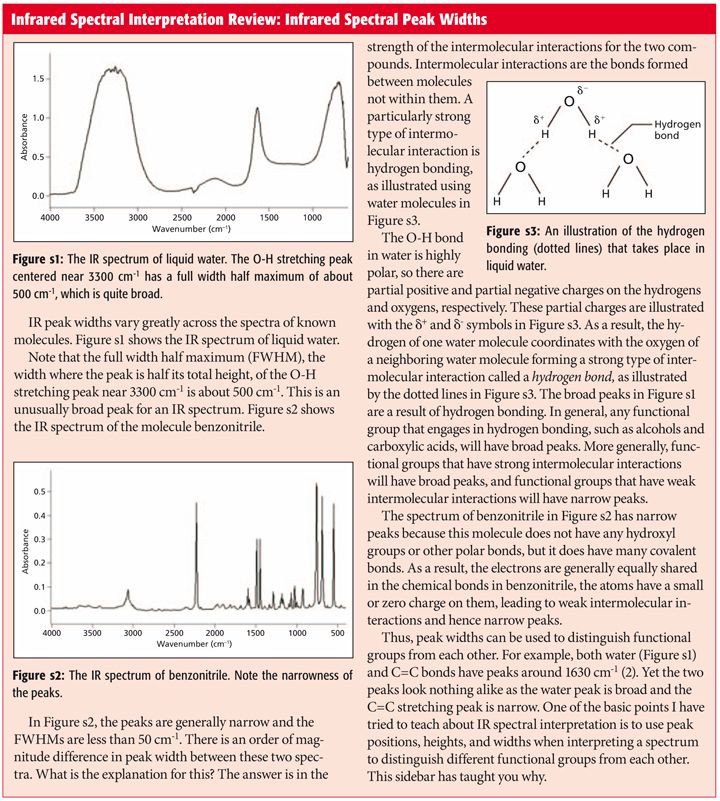
The C O Bond Part Iii Carboxylic Acids
The C O Bond Part Iii Carboxylic Acids

Infrared Spectrum Of Butanoic Acid C4h8o2 Ch3ch2ch2cooh Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Butyric Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts
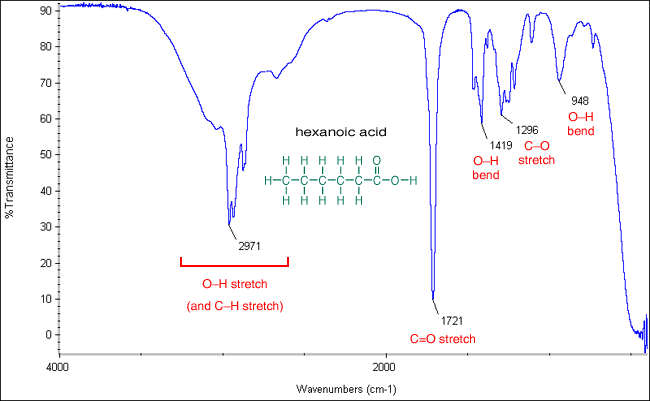

Comments
Post a Comment